Monday-Friaday-8:00am-5:00pm | 0800 600 216 (Toll Free)

The Medicines Regulatory Authority (“Authority”) is a corporate body established under Section 3 of the Medicines and Related Substances Act (“MRSA”) and referred to as the Botswana Medicines Regulatory Authority or BoMRA. The Authority is responsible for ensuring the safety, efficacy, and quality of medicines and related substances, including human and veterinary medicines, medical devices, and cosmetics in Botswana.
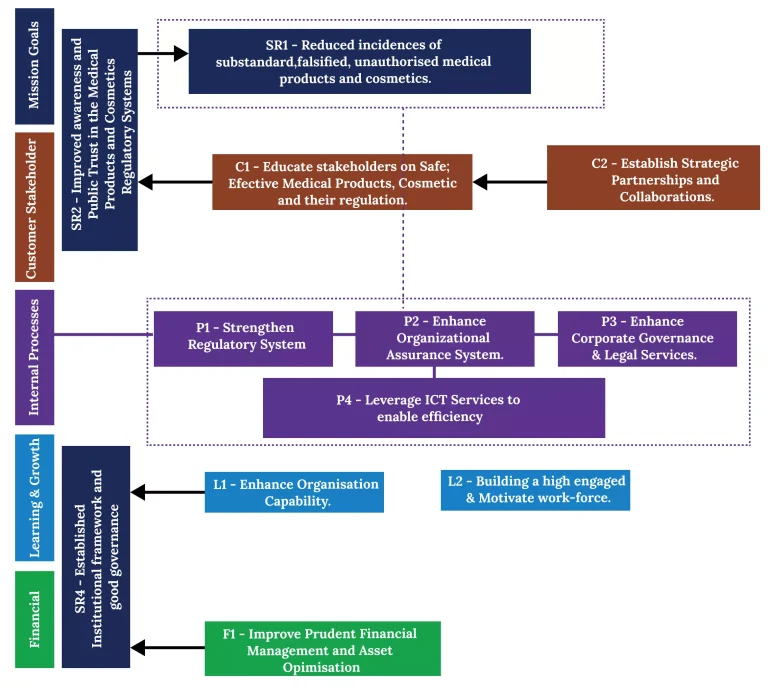
We aim to strengthen corporate governance and good Regulatory Practices by upholding relevant governance codes.
Improve Corporate Governance & Performance
We aim to preserve a positive culture that prioritizes performance and results
Enhance Results-Oriented Culture
We seek to have skilled employees in all strategic positions to drive regulatory business
Develop A Talented and Engaged Workforce
We aim to achieve both financial sustainability and operational independence
Achieve Financial Sustainability and Asset Optimisation
we seek to leverage digital technology and innovation to grow business
Drive Digital Transformation
We seek to strengthen consumer education and gather feedback to better align with customer needs and expectations
Promote Patient/Animal Safety
We aim to build strong and effective relationships with diverse stakeholders
Develop Strategic Partnerships
We seek to optimize all key regulatory processes to enhance efficiency and effectivenessons
Strengthen Regulatory System
We seek to focus on improving stakeholder satisfaction to ensure that service provision is accessible, responsive and seamless.
Attain Stakeholder Trust and Confidence
We aim to build strong leadership that addresses identified gaps and drives strategic objectives
Build Leadership Capability

Scan here for location:

Plot 112, Gaborone International Finance Park Gaborone, Botswana
Private Bag 2 Gaborone Station Botswana
© 2025 Botswana Medicines Regulatory Authority. All Rights Reserved.
Powered By Atom Media – Unleashing Digital Excellence